Prime Defense™ Surgical Respirators
NIOSH Approved

Foldable N95 Surgical Respirator
F-01S
- NIOSH Surgical Rated Approved
- ≥95% BFE (Bacterial Filtration Efficiency) according to ASTM F2101
- ≥95% Filter Efficiency according to ICS Laboratories Inc.
- Lightweight construction promotes longer wear time
- Adjustable nose clip will help provide custom fit and secure seal
- One size fits most
- Disposable, single use, non-sterile
- Latex free: Yes
- Model: Foldable
Package Information:
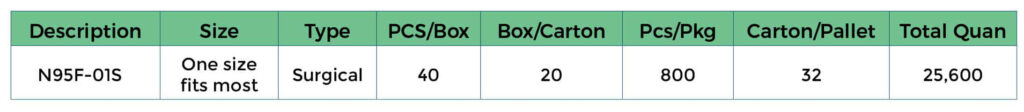

Molded N95 Surgical Respirator
C-02S
CS-02S
- NIOSH Surgical Rated Approved
- ≥95% BFE (Bacterial Filtration Efficiency) according to ASTM F2101
- ≥95% Filter Efficiency according to ICS Laboratories Inc.
- Pre-formed for secure fit
- Adjustable nose clip will help provide custom fit and secure seal
- Available in Small and Regular
- Disposable, single use, non-sterile
- Latex free: Yes
- Model: Cup
Package Information:
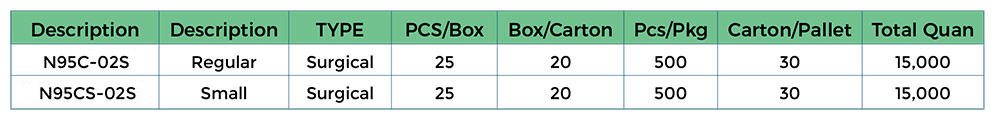

Foldable N95 Surgical Respirator with Moldable Nose Clip
F-07S
- Meets NIOSH inhalation procedure testing TEB-APR-STP-0007
- 99% BFE (Bacterial Filtration Efficiency) according to ASTM
- 7% Filter Efficiency according to ICS Laboratories Inc.
- Berry Amendment (10 S.C. 2533a) compliant
- Lightweight construction promotes longer wear
- Moldable nose clip will help provide custom fit and secure
- One size fits most
- Disposable, single use, non-sterile
- Latex free: Yes
- Model: Foldable
Package Information:

Use Instructions
- Before occupational use for respiratory protection, a written respiratory protection program must be implemented meeting all requirements of OSHA 29 CFR 1910.139 and/or 1910.134 such as medical evaluation, training and fit testing. In Canada, CSA standard Z94.4 requirements must be met.
- Respirator may be used until damaged, breathing becomes difficult, or contaminated with blood or body fluids.
- It is recommended to use the respirator for an extended period rather than reusing. If reusing the respirator, ensure you inspect for damage, contamination from blood or body fluids, and consult with your organizations policies on reusing respirators. It is not recommended to reuse the respirator.
- Filtering facepieces are to be inspected prior to each use to assure there are no holes in the breathing zone. Enlarged holes resulting from ripped or torn filter material is considered damage. Immediately replace respirator if damaged.

- Cup the mask into your hand with the nosepiece right-side up and the headbands hanging down
- Position the respirator under your chin and the nosepiece on the bridge of your nose
- While holding the respirator in position, pull the top headband over your head and rest it at the crown of the back of your head
- As you continue to hold the bottom headband over your head and rest it around your upper neck, below your ears
- Untwist the straps and optimally position the respirator low on your nose
- Using both hands, mold the nosepiece to fix snugly against the nose bridge and face, below the eyes
- Using a mirror, further check and adjust the edges to provide a good seal around the face
- Perform a user seal check prior to each wearing. To check the respirator-to-face seal, cover the mask with one or both hands, Inhale and exhale sharply. Be careful not to disturb the position of the respirator. If air leaks around the nose, re-adjust the nosepiece as described in step 6. If air leaks around respirator edges, adjust panels and straps. If you CANNOT achieve a proper fit, DO NOT enter the contaminated area. See your supervisor.

Breathe Medical Manufacturing Ltd.
British Columbia
sales@breathemedicalmanufacturing.com
